Three ongoing clinical trials of ELEVIDYS (delandistrogene moxeparvovec-rokl), a gene therapy developed by Sarepta and marketed by Roche, have been temporarily halted by the European Medicines Agency (EMA). This expected but not unwelcome news has caused deep sadness in the Duchenne muscular dystrophy (DMD) community.
The paused trials include the Sarepta-sponsored phase 3 Envision study, which is evaluating Elevidys in boys aged 8 to 17; Roche’s phase 2 Envol study, which is investigating Elevidys in babies and newborns; and Sarepta’s Study 104, a phase 1 look at Elevidys in boys ages 4 to 9 with pre-existing antibodies to a particular serotype.
Before Roche, Sarepta and EMA the first community to publish this announcement was our Turkish partner >>> DMD Dayanisma.
Roche has issued a letter to the community which you can read here. (PDF)
Table of Contents
Why Were Elevidys Clinical Trials Halted?
After the death of a 16-year-old boy with Duchenne muscular dystrophy who received an infusion of Elevidys on March 18, 2025, the outlook for the gene therapy, which is marketed at a commercial price of $3 million, changed abruptly. (Read More)
Until the precise cause of death of a U.S. teen who developed acute liver failure (ALF) after taking Elevidys can be ascertained, the European Medicines Agency (EMA) requested that the studies be put on hold. When the patient passed away last month, Sarepta declared that it will amend Elevidys’ label to include the safety signal.
SRP-9001-104 Temporarily Halted
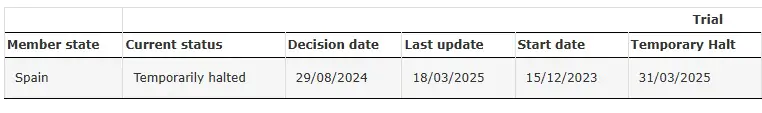
Source: Clinical Trial of SRP-9001-104
ENVOL (Study 302) Temporarily Halted
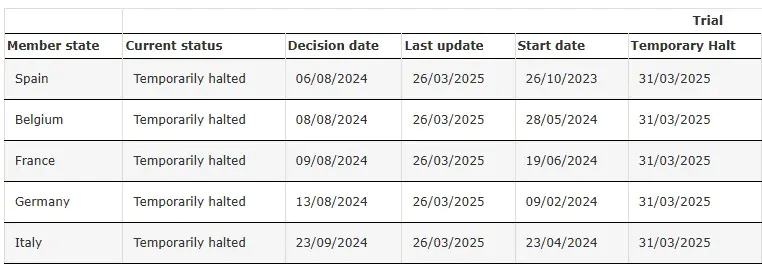
Source: Clinical Trial of SRP-9001-302
SRP-9001-303 Temporarily Halted
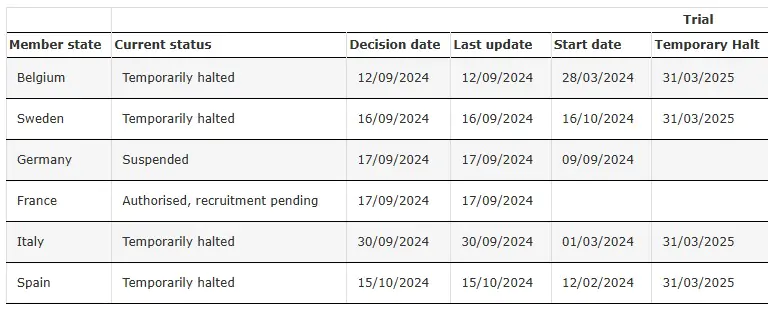
Source: Clinical Trial of SRP-9001-303
Learn More: DMD WarrioR’s Turkish Representative Shares His Views on Elevidys Gene Therapy.




https://investorrelations.sarepta.com/news-releases/news-release-details/sarepta-therapeutics-provides-update-elevidys
Sarepta Therapeutics Provides (safety) Update on ELEVIDYS
04/04/25 8:44 AM EDT
CAMBRIDGE, Mass.–(BUSINESS WIRE)–Apr. 4, 2025– Sarepta Therapeutics, Inc. (NASDAQ:SRPT), the leader in precision genetic medicine for rare diseases, shared the following update related to ELEVIDYS (delandistrogene moxeparvovec-rokl), the only approved gene therapy in patients with Duchenne muscular dystrophy.
Following the safety update on acute liver failure that was issued on March 18, European Union (EU) reference member country authorities requested that the independent data monitoring committee (DMC) meet to review the adverse event. While the analysis is being finalized, recruitment and dosing in certain clinical studies of ELEVIDYS are temporarily halted.
The independent DMC met on April 3 and concurred that based on the totality of evidence, the overall benefit-risk profile remains favorable to continue dosing in the paused clinical trials without changes to the study protocols. At the request of EU regulators, Sarepta and Roche will submit this information in a response to the temporary halt within a week. Evaluation of the submission and the subsequent decision for lifting the temporary halt will follow the EU regulatory process.
The clinical studies affected by the temporary halt are Study SRP-9001-302 (ENVOL), Study SRP-9001-303 (ENVISION) and Study SRP-9001-104. Monitoring and data collection for already-enrolled participants continues, and we do not anticipate a material impact on the timeline for these studies.
Stocks fell on the stock market.
https://dmdwarrior.com/dmd-warriors-turkish-representative-shares-his-views-on-elevidys-gene-therapy-is-it-effective-and-why-is-it-expensive/