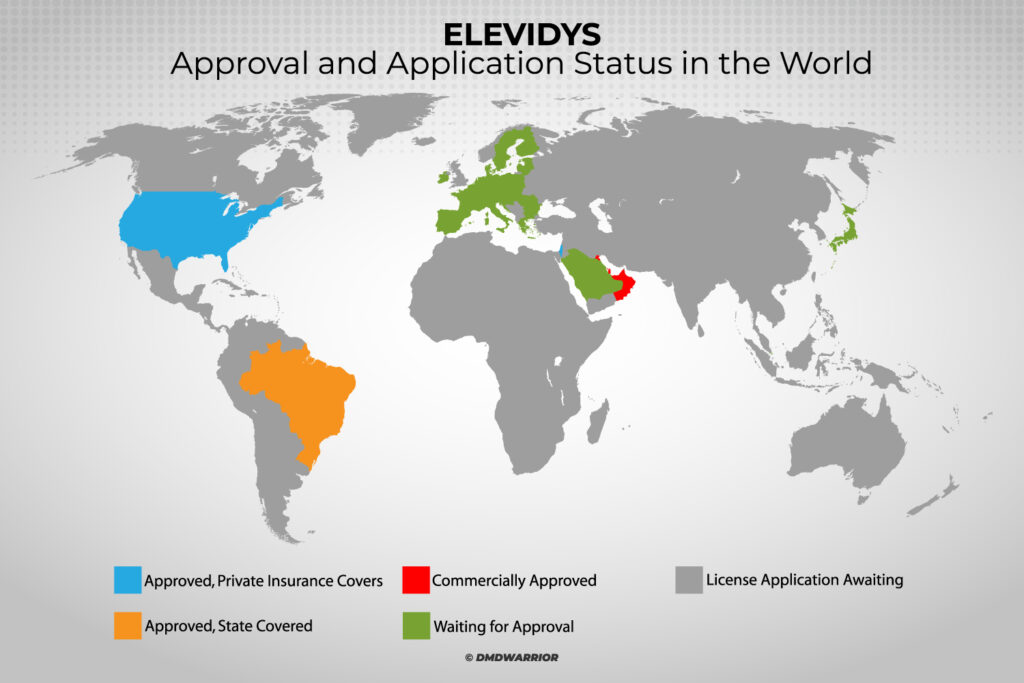
Elevidys is the only approved gene therapy in the World for Duchenne muscular dystrophy and received accelerated approval in the US in June 2023, and is now approved in the United Arab Emirates, Qatar, Kuwait, Bahrain, Oman, Brazil and Israel for the treatment of ambulant children aged 4 through 5 years with Duchenne, who have a confirmed mutation in the DMD gene. [Read More: Roche announced that EMA has initiated review of the ELEVIDYS]
Sarepta Therapeutics announces expansion of US FDA approval in 2024 for Elevidys for Duchenne Muscular Dystrophy patients ages 4 and older.
Table of Contents

FDA Expanded Approval of Gene Therapy for Patients with Duchenne Muscular Dystrophy
After this announcement, U.S. Food and Drug Administration expanded the approval of Elevidys (delandistrogene moxeparvovec-rokl), a gene therapy for the treatment of Duchenne muscular dystrophy (DMD) for ambulatory and non-ambulatory individuals 4 years of age and older with DMD with a confirmed mutation in the DMD gene. [Read More: Sarepta Wins Full Approval and Label Expansion for DMD]
In Which Countries Is Elevidys Approved?
Elevidys gene therapy now approved in the US, United Arab Emirates, Qatar, Kuwait, Bahrain, Oman, Brazil and Israel.
Will EMA Not Approve Elevidys?
The European Medicines Agency (EMA) requested a temporary halt to Studies 104 (NCT06241950), Study 302 (ENVOL, NCT06128564) and Study 303 (ENVISION, NCT05881408) pending the completion of their investigation due to the death of a 16-year-old child who received Elevidys gene therapy. (Read More: Study 104, Study 302 and Study 303 were temporarily halted in Europe)
Discover More: Duchenne Muscular Dystrophy: Treatment & Cost
Is Elevidys Approved In Europe?
European Medicines Agency committee ruled that the therapy, called Elevidys, failed to show in studies that it improved patients’ movement abilities. The European Medicines Agency has recommended the refusal of the marketing authorisation for Elevidys, a medicine intended for the treatment of Duchenne muscular dystrophy. – Read More: The European Medicines Agency (EMA) Issued a Negative Opinion on Elevidys –
Applications for approval are currently under review in Switzerland, Singapore, Hong Kong and Saudi Arabia. [Read More: Roche]
Learn More: Frequently Asked Questions About Elevidys Used for Duchenne Muscular Dystrophy



