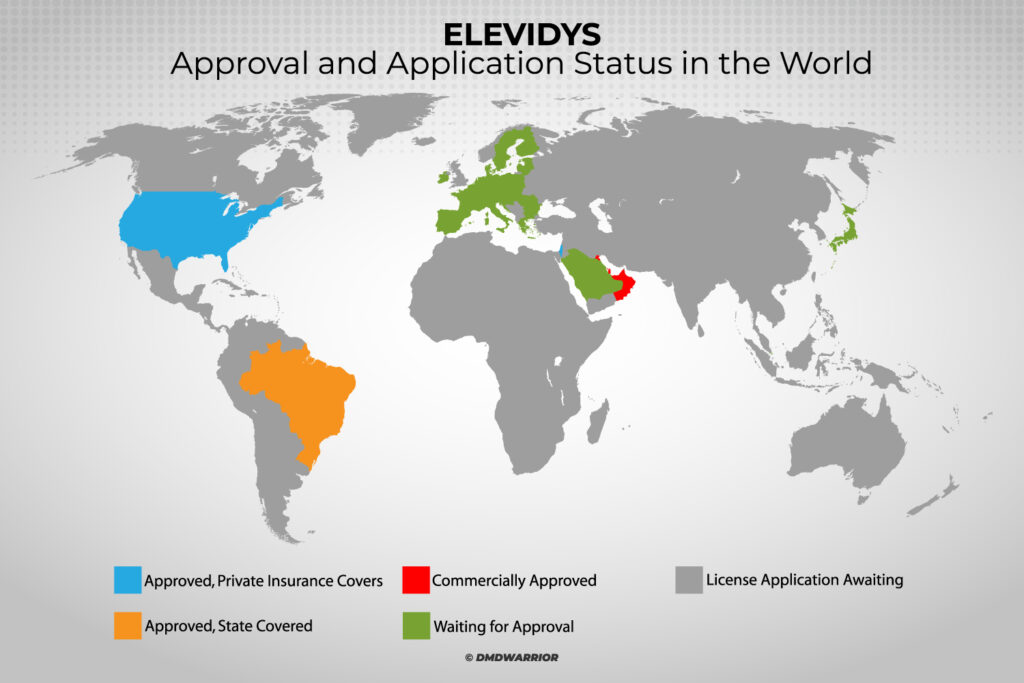
Gene therapies, particularly those like Elevidys, have the potential to revolutionize the treatment of rare and severe genetic disorders, such as Duchenne Muscular Dystrophy (DMD). Elevidys, developed by Sarepta, is a gene therapy designed to address this debilitating condition by replacing the defective gene responsible for DMD with a functional one. Despite its promising results, Roche has been selective about the markets in which it applies for regulatory approval, raising questions about why it isn’t pursuing marketing authorization in every country.
If you are wondering when Elevidys gene therapy will come to your country, this article may help you get an idea.
Table of Contents
The Complexities of Global Market Expansion
Roche’s decision not to apply for marketing authorization in every country is driven by a combination of commercial, regulatory, and strategic factors. One of the key considerations is the complexity and costs associated with obtaining regulatory approval in different regions. But Roche and Sarepta companies should not hide behind such excuses.
Regulatory agencies around the world, such as the U.S. Food and Drug Administration (FDA), the European Medicines Agency (EMA), and others, have varying standards, timelines, and requirements for gene therapy products. While the FDA and EMA have made considerable strides in expediting the approval process for gene therapies, other countries may lack similar frameworks, making the approval process slower, more expensive, and uncertain.
Additionally, regulatory approval in certain regions may require extensive clinical trials or local studies that are not always feasible, especially in countries where the prevalence of the disease is lower or where clinical infrastructure may be lacking. The resources required for such trials can be a deterrent for a company like Roche, which may prioritize markets where it perceives the greatest return on investment.
Commercial Concerns: The High Stakes of Global Licensing
Commercial concerns also play a crucial role in Roche’s decision-making. The cost of gene therapy, especially Elevidys, is extraordinarily high; a single dose of the treatment is reportedly marketed at $3 million.
In countries where reimbursement systems are underdeveloped or where healthcare budgets are constrained, the financial viability of introducing such a therapy may be questionable.
Even in countries with established healthcare systems, such as those in Europe or the U.S., negotiating reimbursement rates can be a lengthy and uncertain process.
In many cases, insurance companies and governments are hesitant to approve coverage for therapies with such a high upfront cost, especially when the long-term benefits may be difficult to quantify.
Roche is also considering the market size in each country.
Duchenne Muscular Dystrophy, while devastating, affects a relatively small patient population in any given region. In countries where DMD is less prevalent, the cost of bringing Elevidys to market may not justify the potential return on investment.
Roche may prefer to focus on larger markets with higher patient populations or more favorable reimbursement policies, where the cost of bringing the drug to market is more easily absorbed.
The Impact on Families Seeking Treatment Abroad
The lack of a global marketing authorization for Elevidys has significant consequences for families of children suffering from Duchenne Muscular Dystrophy, especially in countries where the treatment is not available.
Families facing limited treatment options are often left with no choice but to seek treatment abroad, where the therapy may be approved. This situation places an immense financial burden on patients and their families.
The high cost of Elevidys, coupled with the additional expenses of traveling abroad—such as medical travel, accommodation, and other logistical costs—can be prohibitive. For many families, the choice to seek treatment in another country may result in financial hardship or even bankruptcy.
Moreover, the emotional and physical toll of traveling for life-saving treatment can exacerbate the already challenging situation faced by patients and their families.
While some governments and nonprofit organizations are working to subsidize the cost of medical travel, these efforts are often insufficient to cover the full range of expenses involved.
Families may also struggle with the uncertainty and stress of navigating foreign healthcare systems, which can vary significantly from their home country.
Furthermore, the lack of local treatment options can create a sense of inequity, as only those who are financially able to seek treatment abroad will have access to potentially life-saving therapies like Elevidys.
How Effective Is Elevidys?
Sarepta has already stated that its Elevidys gene therapy is not a definitive cure.
When clinical studies are examined, it can be seen that it does not produce very high levels of dystrophin, and its North Star Ambulatory Assessment (NSAA) scores are not as impressive as expected. [Read More]
Is Elevidys Price Reasonable Based on Clinical Trial Results?
In our opinion, no.
So why is Elevidys gene therapy so expensive? [Read More]
Many DMD patients and their families believe that the reason Elevidys is so expensive is because companies want to make a profit.
For this reason, patients and families who still have time are eagerly waiting for alternative gene therapies to be approved and brought to market. [New Gene Therapies]
Sarepta and Roche’s high pricing policy undermines trust in these companies and gene therapy.
High Earnings from Individual Campaigns
Are Roche and Sarepta pushing DMD patients to campaign individually?
Many families, whose country has not yet approved the Elevidys gene therapy, are trying to collect money by organizing individual campaigns.
A trade similar to the one made in Brazil may not yield high returns. Could this be the reason? [Risk-Sharing Model with Roche]
What do you think about that?
As DMDWarrioR, we have sent emails to both Sarepta and Roche on this issue several times.
We asked why they don’t apply for marketing authorization in every country!
But they did not give us a definitive and convincing answer.
This left us with negative feelings about the exorbitant price of Elevidys and why no application was made.
Conclusion: The Need for Broader Access to Gene Therapy
Roche’s selective approach to applying for marketing authorization for Elevidys in different countries reflects the complexities of the global pharmaceutical market. Regulatory hurdles, high costs, and commercial concerns weigh heavily on the company’s decision-making process. However, the consequences for families seeking treatment are significant, with many left to bear the financial and emotional burdens of seeking care abroad.
To improve access to life-changing therapies like Elevidys, it is essential for both pharmaceutical companies and governments to collaborate on reducing barriers to approval and expanding access to gene therapies. [Read More]
Efforts to standardize regulatory requirements, increase reimbursement options, and support financial assistance for families could help ensure that all patients, regardless of where they live, have an opportunity to benefit from groundbreaking treatments like Elevidys.
In the long term, greater global access to gene therapies could pave the way for more equitable healthcare systems and ensure that life-saving treatments are not limited by geography or financial means.


