The DMD Dayanisma Community, founded by families of children with Duchenne Muscular Dystrophy and followed by many DMD patients in Turkey, wants children who are eligible for Exon 51 skipping to receive their medications. [Read More: Frequently Asked Questions About Eteplirsen (Exondys 51) for DMD Treatment]
According to the information sent to DMD Warrior by DMD Dayanisma Community, it was understood that Eteplirsen (Exondys 51) was included in the scope of payment by the Ministry of Health in the official response given to the written question submitted by Talih Ozcan, a member of parliament in Turkey, regarding the diagnosis, treatment and social rights of Duchenne Muscular Dystrophy (DMD) patients. [Read More: DMD Dayanisma Community]
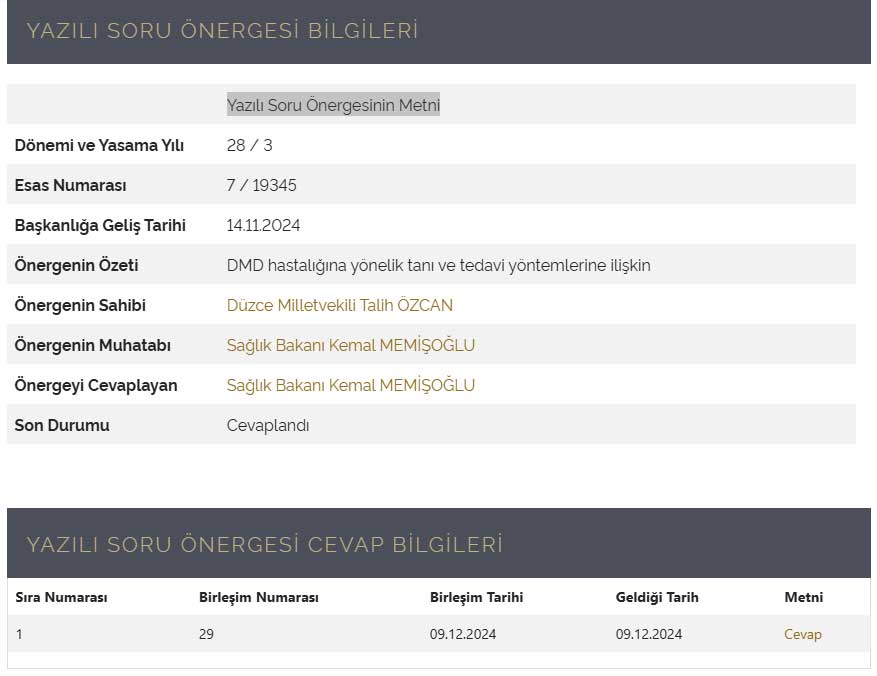
Text of the Written Question Proposal
Response to Text of the Written Question Proposal
Ataluren and Eteplirsen
The Turkish Ministry of Health responded by saying, “For the treatment of Duchenne muscular dystrophy disease, drugs containing the active ingredient Ataluren and Eteplirsen, which are not licensed in our country, applications are made to our institution on a patient basis by physicians within the scope of supplying drugs from abroad. The relevant applications are evaluated by our institution’s Personal Treatment Evaluation Committee and if the use of the drug is deemed appropriate, the drug is imported from abroad through foreign drug suppliers and the patient’s access to the drug is provided.”
According to the DMD Dayanisma Community, patients who are eligible for Exon 51 skipping treatment are not aware that they can get their medication from the Ministry of Health because official associations in Turkey have not made any announcements on their websites about this issue.
A similar answer came to another MP’s written question
The Ministry of Health gave a similar answer to a different question written by Mr. Omer Faruk Gergerlioglu.
While it was stated that Ataluren and Eteplirsen drugs were covered by the ministry, no answer was written regarding ELEVIDYS.
Response to Text of the Written Question Proposal
Questions and answers from other MPs
HATAY MP ADNAN ŞEFİK ÇİRKİN
Qestion:
https://cdn.tbmm.gov.tr/KKBSPublicFile/D28/Y3/T7/WebOnergeMetni/b101fec8-f472-4e16-9d61-71c8b434f39f.pdf
Answer:
https://cdn.tbmm.gov.tr/KKBSPublicFile/D28/Y3/T7/WebOnergeMetni/7f300fa9-cb30-4f74-ac96-2612cce98092.pdf
MUGLA MP SELÇUK ÖZDAĞ
Qestion:
https://cdn.tbmm.gov.tr/KKBSPublicFile/D28/Y3/T7/WebOnergeMetni/44e73af5-333e-49db-913b-f9fc8141d146.pdf
Answer:
https://cdn.tbmm.gov.tr/KKBSPublicFile/D28/Y3/T7/WebOnergeMetni/7c1ca59f-1e20-480c-aac7-a2c65cab6c33.pdf
ANKARA MP DENİZ DEMİR
Qestion:
https://cdn.tbmm.gov.tr/KKBSPublicFile/D28/Y3/T7/WebOnergeMetni/4fa81408-c780-45d5-9640-26e17b98062e.pdf
Answer:
https://cdn.tbmm.gov.tr/KKBSPublicFile/D28/Y3/T7/WebOnergeMetni/0dd13f2d-fcff-4df2-bc79-5db4dfce5cd8.pdf
İSTANBUL MP DOĞAN BEKİN
Qestion:
https://cdn.tbmm.gov.tr/KKBSPublicFile/D28/Y3/T7/WebOnergeMetni/6f7aa69d-4456-450c-9e68-2f5802704d44.pdf
Answer:
https://cdn.tbmm.gov.tr/KKBSPublicFile/D28/Y3/T7/WebOnergeMetni/989d106c-ff18-46ed-a112-220065f9ee15.pdf
İSTANBUL MP CELAL FIRAT
Qestion:
https://cdn.tbmm.gov.tr/KKBSPublicFile/D28/Y3/T7/WebOnergeMetni/80a4deb6-a24a-42c0-8489-3de01989cfaa.pdf
Answer:
https://cdn.tbmm.gov.tr/KKBSPublicFile/D28/Y3/T7/WebOnergeMetni/c5adb248-7eb0-448a-9972-b2cf7c990189.pdf
Also Read This: In Brazil, the Government is Expected to Implement a Risk-Sharing Model with Roche for ELEVIDYS