Satellos Bioscience, a clinical-stage biotechnology company focused on developing regenerative therapies for muscle diseases, has announced at ClinicalTrials.Gov it is preparing to initiate a Phase 2 clinical study of its lead drug candidate, SAT-3247, targeting Duchenne Muscular Dystrophy (DMD). This marks a significant milestone in the company’s mission to offer a transformative treatment for individuals affected by this rare and debilitating genetic disorder.
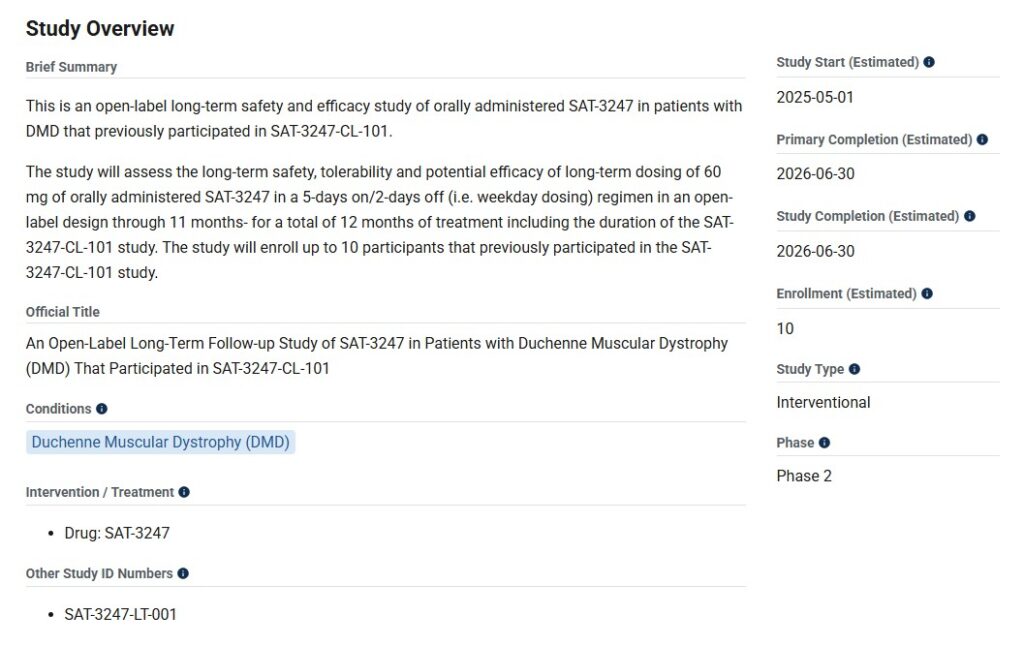
Satellos SAT-3247 Phase 2 Clinical Study
What is SAT-3247?
SAT-3247 is a first-in-class small molecule therapy designed to restore the body’s ability to regenerate muscle by targeting muscle stem cell polarity. Unlike current DMD therapies that primarily aim to slow muscle degeneration, SAT-3247 focuses on activating the body’s natural regenerative pathways to rebuild and repair damaged muscle tissue—offering a potentially groundbreaking approach to disease modification.
Learn More: Satellos SAT-3247 Stem Cell Approach May Restore Muscle Regeneration in Duchenne Muscular Dystrophy
Why This Matters for Duchenne Muscular Dystrophy
Duchenne Muscular Dystrophy is a progressive genetic condition that primarily affects boys, causing muscle weakness, loss of mobility, and premature death. Current treatments are limited, and many focus on symptom management rather than halting or reversing the course of the disease.
The launch of Phase 2 clinical trials for SAT-3247 is a critical step forward in addressing this unmet medical need. According to Satellos, the goal is to evaluate the drug’s safety, tolerability, and efficacy in a broader group of DMD patients following promising Phase 1 results.
What’s Next for the Clinical Trial?
Satellos has indicated that it is finalizing preparations for trial site activation and patient recruitment. The Phase 2 trial will be conducted across St. Vincent Hospital in Australia and will include maless aged 18 to 40 with confirmed DMD diagnoses. The study will also assess biomarkers of muscle regeneration and functional improvements, potentially setting a new benchmark in the treatment landscape for Duchenne.
Dr. Frank Gleeson, CEO of Satellos, stated, “We are thrilled to move SAT-3247 into Phase 2. This step reflects our commitment to pioneering a regenerative medicine approach for Duchenne and improving the lives of patients and families affected by this devastating disease.”

Learn More: Satellos SAT-3247 Phase 2 Clinical Study: NCT06867107
A New Chapter in Muscle Regeneration
With SAT-3247, Satellos is not only pushing the boundaries of scientific innovation but also delivering hope to thousands of families worldwide. If successful, this therapy could usher in a new class of regenerative treatments for muscular dystrophies and related conditions.
How can My Son Participate in the SAT-3247 Phase 2 Clinical Trial?
Although Satellos has announced that the SAT-3247 Phase 2 clinical trials will be conducted in Australia, we recommend that DMD patients who have the opportunity to travel to Australia and meet the criteria contact Satellos using the contact details below.
Name: Satellos Medical Affairs/Clinical Development
Phone Number: +61 3 8736 1750
Email: [email protected]
About Satellos Bioscience
Satellos Bioscience is a Toronto-based biotechnology company focused on discovering and developing novel regenerative medicines for diseases involving muscle degeneration. Leveraging its proprietary MyoReGenX™ platform, Satellos aims to unlock muscle stem cell biology to reverse the progression of muscular dystrophies.



Soy paciente femenina de 59 años con distrofia muscular del anillo óseo y me gustaría participar en este proyecto
Se seleccionará entre los participantes del estudio de Fase 1.
Excelente ojalá salga todo bien el ensayo así pueda mejorar la vida de muchos chicos soy norma mamá de un chico con DMD
Hola cómo puedo anotar a mi hijo para el ensayo clínico ?
Este es un estudio abierto de seguridad y eficacia a largo plazo de SAT-3247 administrado por vía oral en pacientes con DMD que participaron previamente en SAT-3247-CL-101. Se seleccionará entre los participantes del estudio de Fase 1.
Hola, mi nombre es Nadim tengo 24 años tengo distrofia duchene, como podria anotarme??
Usted tiene la edad elegible para participar en el estudio, pero este ensayo clínico actualmente solo está disponible en Australia.
Tengo la posibilidad de ir a Australia, donde me tendría que anotar ??muchas gracias
Puedes contactar con Satellos con la siguiente información.
Name: Satellos Medical Affairs/Clinical Development
Phone Number:
+61 3 8736 1750
Email: [email protected]
Hola, estamos muy interesados en esta medicación. Nuestro hijo cumple con los criterios de inclusión en el ensayo de fase dos. Estamos en Argentina y disponibles a viajar a Australia. hablar con alguien por teléfono en español?
Puede ponerse en contacto con Satellos directamente utilizando la información de contacto proporcionada anteriormente. No estamos seguros de si alguien habla español. Por lo tanto, le recomendamos que se ponga en contacto con Satellos por correo electrónico.