Duchenne muscular dystrophy (DMD) is rooted in the intricate world of genetics, specifically emanating from changes in a single gene known as the Dystrophin gene.
This gene plays a critical role in muscle function by producing dystrophin, a protein essential for maintaining healthy muscle fibers.
When mutations occur within this gene, it can lead to the progressive weakening and degeneration of muscles characteristic of Duchenne.
While DMD is most commonly inherited from carrier mothers who pass on the abnormal gene to their sons, approximately one-third of cases arise spontaneously due to random genetic mutations that may happen during early embryonic development—thus affecting families with no prior history of the disease.
The implications are profound: siblings or cousins may find themselves grappling with similar challenges despite no evident familial links to Duchenne’s legacy.
Understanding these genetic causes of Duchenne muscular dystrophy not only sheds light on its transmission patterns but also highlights how this condition can ripple through family trees unexpectedly, leaving an indelible mark on those affected and their loved ones alike.
What is the role of dystrophin?
Duchenne muscular dystrophy (DMD) is primarily driven by genetic causes, specifically a mutation in the dystrophin gene. This gene encodes for a crucial protein known as dystrophin, which serves as an essential component in maintaining the structural integrity and proper functioning of muscle cells.
Think of dystrophin as a vital “shock absorber” within muscle fibers; it withstands the stresses that occur during everyday movements, ensuring muscles can contract and relax without succumbing to damage.
In individuals with DMD, however, the absence or malfunctioning of this protein leads to significant vulnerabilities. The lack of functional dystrophin results in increased susceptibility of the muscle cell membrane to damage from ordinary activities—tiny micro-tears begin to form with each contraction and relaxation cycle.
These breaches allow excess calcium ions to flood into the cells; while calcium plays many beneficial roles in cellular processes, its unchecked entry becomes toxic for muscle tissue. This inundation accelerates cellular injury and death, paving the way for degenerative changes where healthy muscle fibers are replaced by scar tissue and fat deposits over time.
As these processes unfold relentlessly underpinned by genetic factors at play, individuals affected by DMD experience progressive loss of strength and function that severely impacts their mobility and quality of life.

Genetic causes of DMD (Duchenne Muscular Dystrophy) are primarily rooted in mutations within the dystrophin gene, which is notably one of the largest genes in humans.
These mutations can manifest as deletions, duplications, or point mutations that alter the structure and function of the dystrophin protein integral to muscle cell integrity.
In healthy individuals, dystrophin acts like a stabilizing anchor between muscle fibers and their surrounding cellular environment; however, when these genetic alterations occur—leading to insufficient amounts or improperly functioning dystrophin—the result is progressive muscular degeneration characteristic of Duchenne.
Specifically, certain types of mutations disrupt the production entirely or render it ineffective for maintaining muscle cell stability and resilience against contraction-induced damage.
Interestingly, while Duchenne arises from more severe disruptions leading to a complete absence of functional dystrophin in muscle cells, Becker muscular dystrophy results from less severe mutations that allow for some residual functionality.
The vast array of reported mutations—totaling thousands—highlights how frequently this large gene acquires changes over time due to its size alone; yet it’s crucial to note that these genetic variations arise naturally rather than through any external influence or intentional action on anyone’s part.
Each individual carries numerous benign gene variants throughout their genome without ever being aware they exist—a testament to both human diversity and complexity at the molecular level.
How is Duchenne inherited?
How is Duchenne inherited? Understanding the intricacies of sex chromosomes provides key insights into this question.
In humans, females possess two X chromosomes, while males have one X and one Y chromosome.
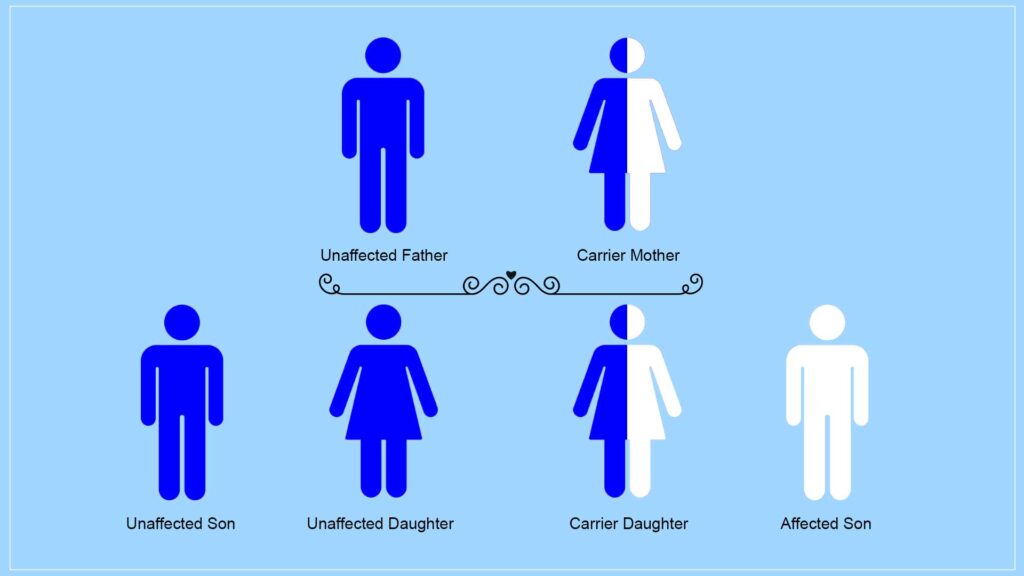
The inheritance process begins with each parent contributing one chromosome from their respective pairs to their offspring—thus, children inherit half of their genetic material from their mother and half from their father.
Males uniquely receive their X chromosome solely from their mother and the Y chromosome exclusively from their father, establishing a clear path for how certain traits are passed down through generations.
For females, they inherit one X chromosome from each parent; however, it is crucial to note that when it comes to conditions like Duchenne muscular dystrophy—a severe muscle-wasting disorder—the gene responsible lies on the X chromosome.
This means Duchenne is classified as an X-linked condition. As a result, women who carry a mutation in this gene can pass it along primarily to their sons during reproduction since males are at greater risk due to having only one copy of the X chromosome—if that single copy contains the mutation associated with Duchenne muscular dystrophy, they will express the disease’s symptoms. Thus, understanding these genetic principles elucidates how Duchenne can be transmitted across generations within families.
Why does Duchenne (DMD) primarily affect males?
Duchenne muscular dystrophy (DMD) primarily affects males due to the distinct genetic inheritance associated with the X chromosome.
In females, possessing two copies of the X chromosome provides a valuable safeguard against certain genetic disorders; if one copy carries a mutation in the dystrophin gene—critical for muscle function—the second, normal copy often compensates, preventing the onset of symptoms.
This redundancy is absent in males, who carry only one X chromosome paired with a Y chromosome. When this sole X chromosome harbors a mutation in the dystrophin gene, as seen in DMD or its milder variant Becker muscular dystrophy (BMD), there is no backup mechanism to mitigate its effects.
Consequently, affected boys manifest significant muscle weakness and degeneration at an early age compared to their female counterparts who may be carriers without ever exhibiting clinical signs of these debilitating conditions. Thus, it’s this disparity in genomic architecture—not merely chance—that elucidates why Duchenne and Becker are predominantly observed among male individuals.



