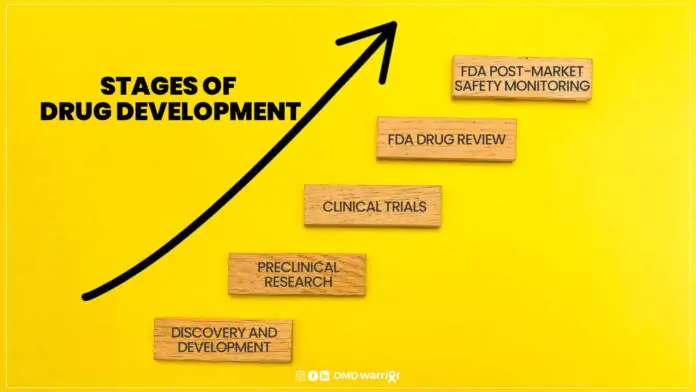
The process of drug development is a highly intricate, lengthy, and costly journey that spans many years, typically between 10 to 15 years, from initial discovery to market launch. Including cures for Duchenne Muscular DystrophyIt involves several stages, each carefully designed to evaluate the safety, efficacy, and potential risks of a new drug before it can be made available to the general public. The stages of drug development are broken down into preclinical research and clinical trials, which are the primary focus of regulatory agencies like the Food and Drug Administration (FDA) and the European Medicines Agency (EMA).
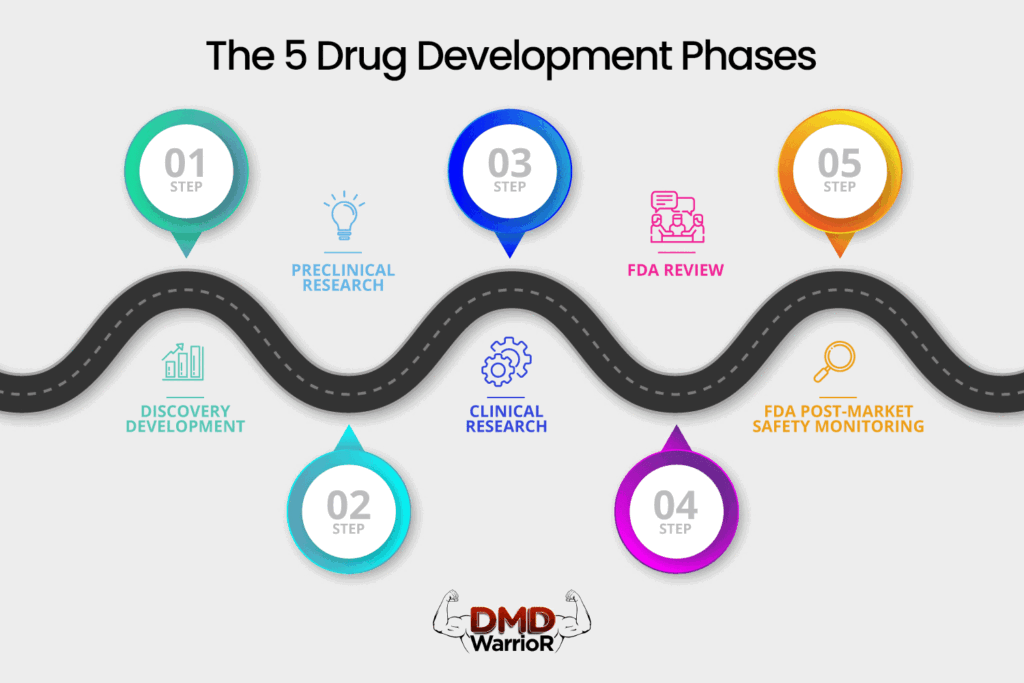
A new medication must pass through five distinct stages in order to be considered a “success”: 1) Discovery and Development; 2) Preclinical Research; 3) Clinical Trials; 4) FDA Drug Review; and 5) FDA Post-Market Safety Monitoring. [FDA]
Table of Contents
Discovery and Development
Before a drug even enters the clinical trial phase, there is a significant amount of preliminary research that occurs. This phase includes:
- Drug Discovery: The initial step involves identifying a biological target (such as a protein or gene) that is involved in a disease. Scientists may screen large libraries of compounds to identify candidates that could affect the target. These compounds are then optimized to improve their efficacy and reduce potential side effects.
Preclinical Research
- Preclinical: Once a promising compound is identified, it undergoes laboratory tests in vitro (outside of a living organism) and in vivo (in living organisms, typically animals) to assess its toxicity, pharmacokinetics (how the drug is absorbed, distributed, metabolized, and excreted in the body), and pharmacodynamics (how the drug affects the body). These studies help determine whether the drug is likely to be safe and effective in humans.
- Toxicology Studies: During preclinical research, scientists conduct detailed studies to assess any potential harmful effects of the drug. The goal is to identify the maximum tolerated dose and any adverse effects that could occur.
If preclinical studies are successful, the drug moves on to the clinical trial phase, which involves testing the drug in humans.
Clinical Trials
What are phase 1, phase 2, phase 3 clinical trials?
The clinical trial process is divided into four distinct phases (Phase 1, Phase 2, Phase 3, and Phase 4) to progressively evaluate a drug’s safety, efficacy, and overall benefit-risk ratio in humans. Each phase serves a unique purpose in the evaluation process.
Phase 1: Safety and Dosage
Phase 1 is the first time the drug is tested in humans after preclinical research. The main goal of Phase 1 trials is to assess the drug’s safety profile, determine a safe dosage range, and identify any side effects.
- Study Design: Typically, Phase 1 trials are small, involving 20 to 100 healthy volunteers or people with the disease/condition. These trials are usually conducted in a controlled clinical environment like a hospital or research facility.
- Objectives:
- Safety: Researchers monitor the volunteers closely for any adverse effects, ranging from mild symptoms to more serious reactions.
- Dosage: The initial dosing of the drug is often conservative. The drug is gradually escalated in a process known as dose-escalation to determine the maximum tolerated dose.
- Pharmacokinetics and Pharmacodynamics: Researchers also study how the drug is absorbed, distributed, metabolized, and excreted by the body, as well as its biological effects.
- Duration: Phase 1 trials typically last a few months. If successful, the drug moves on to Phase 2.
Phase 2: Efficacy and Side Effects
Phase 2 trials are conducted to further investigate the drug’s efficacy and safety in a specific patient population who have the disease or condition that the drug is intended to treat.
- Study Design: These trials are typically larger than Phase 1, involving 100 to 300 participants. The focus is on testing the drug’s effectiveness in patients and identifying any adverse effects associated with long-term use.
- Objectives:
- Efficacy: Researchers aim to determine if the drug produces the desired therapeutic effect in patients with the target condition. Efficacy is measured through clinical outcomes, such as improvement in symptoms or disease biomarkers.
- Safety: Similar to Phase 1, researchers continue to monitor safety, but the primary goal is to identify any side effects that may emerge in patients with the disease.
- Optimal Dose: During Phase 2, researchers further refine the drug’s dosing regimen based on the information obtained in Phase 1.
- Duration: Phase 2 trials usually last several months to a few years. If the drug shows efficacy and a favorable safety profile, it progresses to Phase 3.
Phase 3: Confirmatory Trials (Efficacy and Safety)
Phase 3 trials are large-scale studies designed to confirm the drug’s efficacy, monitor its side effects, and compare it to existing treatments or a placebo. This phase plays a critical role in determining whether the drug should be approved for use by regulatory authorities.
- Study Design: Phase 3 trials involve a much larger number of participants, typically ranging from 1,000 to 3,000 or more. These trials are often multi-center, meaning they are conducted across multiple locations and sometimes even internationally.
- Objectives:
- Efficacy: The goal is to confirm that the drug provides a clinically significant benefit over existing treatments or a placebo.
- Safety: Researchers continue to monitor for side effects, but the larger sample size helps identify less common adverse events that may have been missed in earlier phases.
- Comparative Effectiveness: Phase 3 trials often compare the new drug with standard treatments or placebos to demonstrate its superiority or non-inferiority.
- Duration: Phase 3 trials can last several years due to the extensive data collection required. These trials are often the most expensive and time-consuming part of the drug development process.
- End Result: If a Phase 3 trial is successful, the drug sponsor can submit a New Drug Application (NDA) or Biologics License Application (BLA) to regulatory authorities like the FDA or EMA. If approved, the drug moves to the final phase: Phase 4.
FDA Drug Review
A drug compound’s safety and efficacy results from clinical trials are reviewed by a panel of specialists that includes physicians, chemists, statisticians, microbiologists, and pharmacologists once it has passed phase 1, phase 2, phase 3, and phase 4 clinical trials.
A biotechnology or pharmaceutical company must file a Biologics License Application (BLA) for biologics or a New Drug Application (NDA) for medicines in order to request FDA evaluation. After that, the FDA has to approve the application and designate a group of professionals to assess its merits.
The group examines the clinical study together, taking into account the risk-benefit analysis of the medication, possible side effects, and patient results. The FDA authorizes the drug’s manufacture, marketing, and distribution in the US if it is judged safe and effective for its intended use.
FDA Post-Market Safety Monitoring
Once a drug is approved by regulatory agencies, it enters the post-marketing phase, also known as Phase 4. In this phase, the drug is available for use by the general population, but researchers continue to monitor its long-term safety, effectiveness, and any rare or long-term side effects.
- Post-Marketing Surveillance: In Phase 4, ongoing studies and patient reports (such as from adverse event reporting systems) help identify any rare side effects that might not have been detected during earlier trials.
- Real-World Data: Researchers also collect data on how the drug performs in real-world settings and across diverse populations that may not have been adequately represented in clinical trials.
Learn More: Cures of Duchenne (List of All Researches)
Conclusion: The Importance of Each Phase in Drug Development
Each phase of clinical drug development plays a crucial role in ensuring that a new drug is both safe and effective before it reaches the market. From Phase 1, where safety is the primary concern, to Phase 3, which rigorously tests a drug’s overall benefit-risk ratio in a large patient population, and Phase 4, which monitors long-term effects post-market, every phase adds to the understanding of how the drug will impact patients.
The overall drug development process is a complex balancing act, as pharmaceutical companies, regulatory bodies, and researchers work together to bring innovative and life-changing treatments to market while minimizing potential harm. The time, effort, and costs invested in drug development are substantial, but they are essential for advancing medical science and improving patient outcomes across the world.