The Food and Drug Administration published more than 200 letters it sent to companies after their medications were rejected on Thursday, drawing attention to a sometimes overlooked part of the drug review process. One of these rejection letters was sent for Vyondys 53 (golodirsen), a drug manufactured by Sarepta Therapeutics and developed for exon 53 skipping therapy.
The agency focused only on letters to manufacturers whose products were eventually approved, most of which had already been released over the years. By assembling all of the letters in one place, the agency claimed that the activity was a component of a broader effort to promote transparency.
Table of Contents
Details of FDA Rejection Letter About Vyondys 53
The FDA issued its 2019 rejection letter for Vyondys 53 (golodirsen), an exon 53-skipping treatment for Duchenne muscular dystrophy. The FDA stated that given the “small increase in levels of shortened dystrophin” provided by the treatment, the clinical benefit of Vyondys 53 would likely be “commensurately small”.
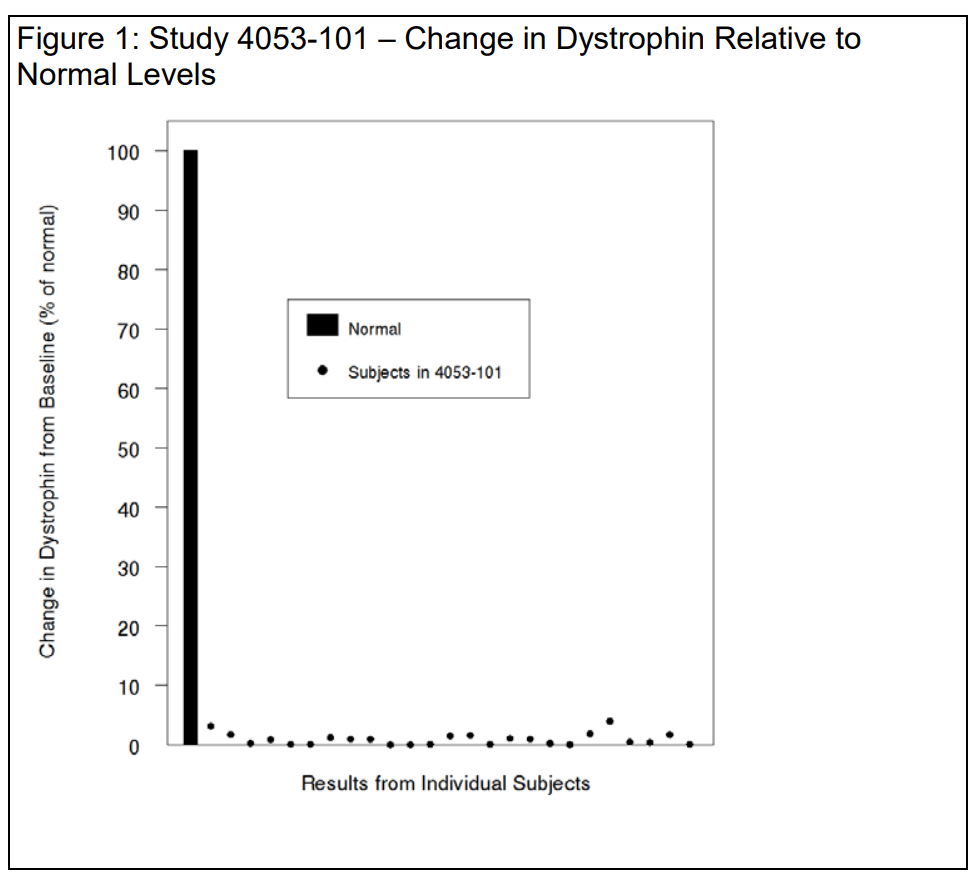
Dystrophin level increased 9 parts in a thousand
The FDA’s rejection letter for Vyondys 53 (golodirsen) noted a very small increase in dystrophin levels after use of the drug:
For the 25 evaluable patients in the study, the baseline mean dystrophin level assessed by Western blot was 0.10 ± 0.07 (percent of normal; mean ± SD). At Week 48, the mean dystrophin level was 1.02 ± 1.03 (percent of normal; mean ± SD), corresponding to a mean increase of 0.92 ± 1.01 percent of normal.
The individual changes in dystrophin are shown below in Figure 1 using a normal (100%) scale to place the data in proper perspective and avoid exaggeration of the effect size. You note that the mean increases in truncated dystrophin are similar in response to golodirsen and eteplirsen in patients with mutations amenable to exon 53 and exon 51 skipping, respectively – with absolute mean increases of 0.9%, i.e., 9 parts in a thousand. We agree.
If one accepts the premise that a small increase in truncated dystrophin, on the order of 9 parts per thousand, is reasonably likely to predict clinical benefit, it seems reasonable to assume that the clinical benefit would be commensurately small.
Clinical Effect of Vyondys 53
The 6-minute walk data from Study 4053-101 are not discussed herein, but they show progressive loss of physical function in essentially all boys. Moreover, there is no correlation between maintenance of physical performance and the magnitude of truncated dystrophin production, further suggesting that if there is indeed a clinical effect of golodirsen, the effect size is small.
Serious Side Effects of Vyondys 53, According to the FDA
In December 2019, the FDA authorized Sarepta’s Vyondys 53 as the first targeted therapeutic option for DMD patients who are susceptible to exon 53 skipping. However, the medicine had been rejected by the FDA four months earlier in a CRL that was signed by Ellis Unger, who was the Center for medicine Evaluation and Research’s Office of Drug Evaluation head at the time.
The FDA stated in its justification for the rejection that, considering the “small increase in truncated dystrophin” that the treatment evoked, the therapeutic effect from Vyondys 53 would probably be “commensurately small.” Unger continued by explaining that there was “no correlation” between the quantity of dystrophin that Vyondys 53 produced in the boys and their physical performance, and that “essentially all” of the boys who were examined after receiving the drug displayed increasing loss of physical function.
As if the bruises weren’t enough, Unger enumerated a long list of safety concerns that Vyondys 53 users had to deal with. These included “renal toxicity” and “serious infections related to drug delivery.” He claims that both “have the potential to be fatal,” with the latter being “difficult or impossible to monitor.”
Given the two recent deaths from severe liver failure linked to Sarepta’s gene therapy Elevidys, this second warning is especially concerning. As Unger noted in the letter, both routes have been linked to hepatic harm, even though Vyondys 53 is an antisense oligonucleotide (ASO) as opposed to an adeno-associated virus (AAV)-based gene therapy like Elevidys.
Read More: Vyondys 53 FDA Rejection Letter
How Was Vyondys 53 Approved?
In order to address the concerns brought up by the regulator in the CRL, Sarepta later filed an appeal and met with the FDA. This finally resulted in the FDA’s Office of New Drugs head at the time, Peter Stein, who left the agency in April, approving Vyondys 53.
Pharmaceutical Companies Don’t Publish All Information
“For far too long, drug developers have been playing a guessing game when navigating the FDA,” said FDA Commissioner Marty Makary, M.D., M.P.H. “Drug developers and capital markets alike want predictability. So today we’re one step closer to delivering it to them, with an ultimate goal of bringing cures and meaningful treatments to patients faster.”
Because the FDA has historically refrained from publishing CRLs for pending applications, sponsors often misrepresent the rationale behind FDA’s decision to their stakeholders and the public. According to a 2015 analysis conducted by FDA researchers, sponsors avoided mentioning 85% of the FDA’s concerns about safety and efficacy when announcing publicly that their application was not approved. Moreover, when FDA calls for a new clinical trial for safety or efficacy, that critical information is not disclosed approximately 40% of the time. Lessons learned from non-approvals are also not shared within the industry, leading companies to repeatedly make similar mistakes. – Read More: FDA Embraces Radical Transparency by Publishing Complete Response Letters –